Question
- Leaving Certificate Chemistry (Higher) 2022: Section A Q3
- Back to the question >
Answer
(a)
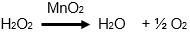
(b) Hydrogen peroxide is highly corrosive to eyes and skin. Always wear safety glasses, lab coat and gloves when using hydrogen peroxide.
(c) Graph showing volume versus time for the decomposition of hydrogen peroxide:
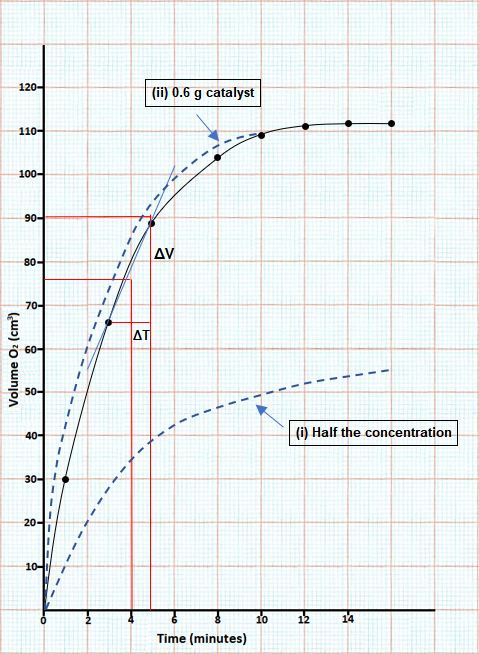
(i) The reaction is complete at 14 minutes.
(ii) 76 cm3 was produced in 4 minutes.
Average rate = 76/4 = 19 cm3/min
(iii) Tangent to the curve is drawn at the point where time = 4 minutes.
Slope =ΔV/ΔT = 18/1.6 = 11.25 cm3/min
(d) (See graph above.)
(i) If the concentration of the hydrogen peroxide is halved then there are less molecules present, which will reduce the rate of the reaction. Therefore, the curve of the graph has a more gradual climb upwards as the gas is released more slowly.
(ii) Changing the mass of the catalyst will have no effect on the rate of the reaction so the curve on the graph will be the same.
