Question
- Leaving Certificate Chemistry (Higher) 2023: Section A Q2
- Back to the question >
Answer
(a)
(i) Preparation of ethyne:
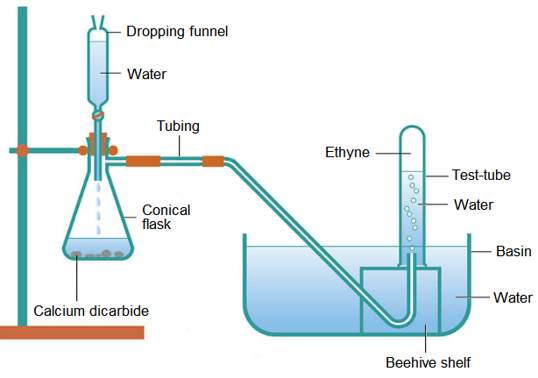
(ii) The first few test-tubes will contain air, which was present in the conical flask.
(b)
(i) Colour change of brown to colourless.
(ii) Colour change of purple to colourless.
(iii) These tests indicate that ethyne is unsaturated.
(c)
(i) Place a lighted wooden splint into the test-tube.
(ii) Ethyne will burn with a luminous, smoky flame and black soot is observed.
(iii)

(iv) When ethyne is burned in the presence of pure oxygen, the flame is not smoky; it is more luminous and there is no black soot formed. This is because the ethyne burns completely to form CO2 and H2O.
(d)
80% of 2.0 = 1.6 g CaC2
Moles of CaC2 = 1.6 / 64 = 0.025 moles
Moles of C2H2 = 0.025
Volume at room temperature = 0.025 x 24 L = 0.6 L
