Question
- Junior Cert. Science SEC Sample: Q15
- Back to the question >
Answer
(a)
Carbon dioxide.(b)
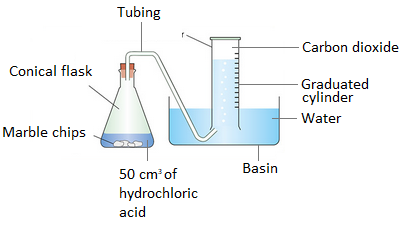
(c)
Place a lighted splint into a conical flask and it will extinguish.
(d)
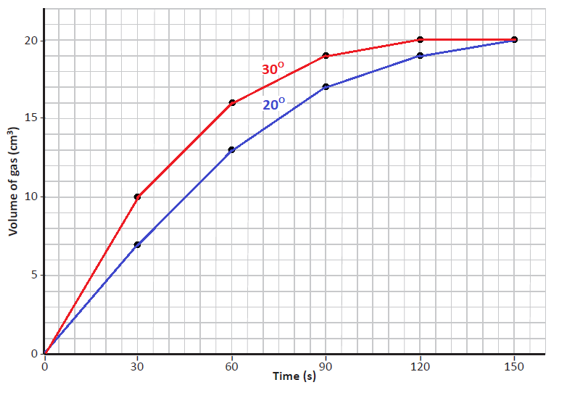

(e)
Conclusions:
- The volume of gas produced at the higher temperature shows a steeper curve, which indicates the reaction is completed in a shorter time than the reaction at the lower temperature.
- Temperature is a factor that can affect the rate of a reaction. The higher the temperature the greater the rate of reaction.
