Question
- Leaving Certificate Physics (Higher) 2020: Section B Q11
- Back to the question >
Answer
(a) J.J. Thomson used cathode ray tubes in his research. How are electrons (i) produced, (ii) deviated in a cathode ray tube?
Electrons are produced by thermionic emission at the cathode. They are deviated when they either pass between charged electric plates known as x- and y-plates, or when they pass through magnetic fields caused by electric magnets.
(b) Cathode rays are accelerated through a potential difference of 4 kV in a cathode ray tube. Calculate the maximum speed of an electron in the tube.
Energy gained:
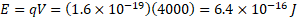
This is kinetic energy:
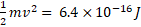
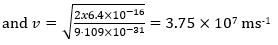
(c) What pieces of apparatus can be used to demonstrate the diffraction of light in the laboratory?
A diffraction grating can be used with a laser to demonstrate diffraction. An interference pattern will be formed on a screen, demonstrating both diffraction and interference.
(d) Geiger also played an important role in the development of the Geiger counter, a detector of nuclear radiation. Describe the principle of operation of any detector of nuclear radiation.
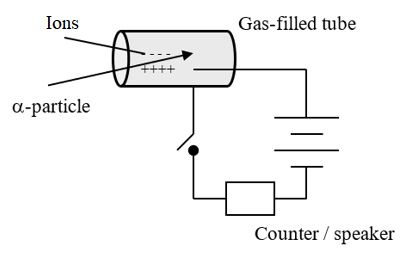
This a metal cylinder containing a mixture of argon and bromine at low pressure. An electric field exists in the tube, as shown.
If ionising radiation should enter the chamber, the ions it creates flow towards either the anode or cathode, and for a few moments a current will flow. The counter can be used to register for how long the current flows, and therefore, how much radiation is present.
(e) Describe the Geiger‐Marsden experiment that used thin sheets of gold. Include their setup, observations and conclusions.
This experiment is usually attributed to Rutherford, with whom Geiger and Marsden were studying.
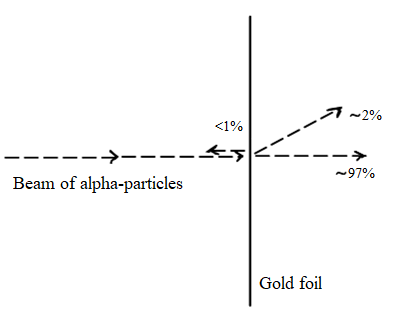
They fired a beam of alpha-particles towards a thin sheet of gold. Alpha particles are small, positively charged particles. Gold was chosen because it can be pressed very thin, so thin that it is only a few atoms from front to back.
Observations and conclusions:
What they found was that most particles went through the foil, without damaging it. A small percentage was diverted as shown, while an even smaller percentage was repelled back towards the source.
This experiment gave rise to the idea of the Rutherford-Bohr model of the atom. In this model,
- Most of the atom is empty space.
- There is a positively charged nucleus, which contains the protons and neutrons.
- The nucleus is surrounded by several 'shells' of negatively-charged electrons.
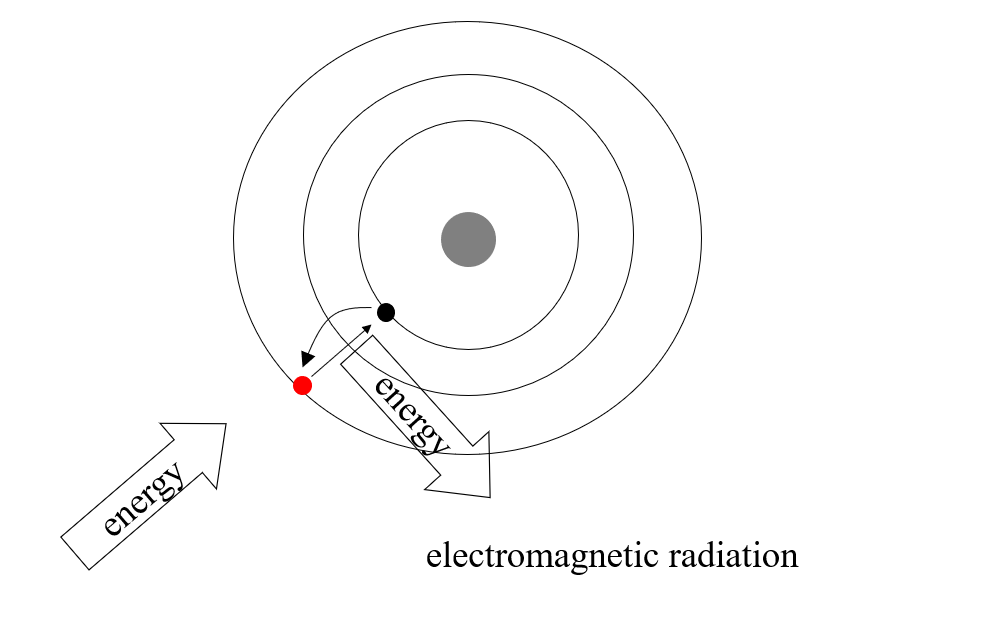
(f) Describe with the aid of a labelled diagram the Bohr model of the atom. Use the model to explain emission line spectra.
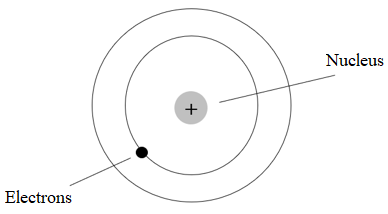
- In their low-energy (ground) state, electrons will occupy a shell as close to the centre of the atom as possible.
- When more energy is available — from heat or light or any other source — an electron will use that energy to move further out from the centre to a higher energy level or shell.
- The electron will stay at the higher level for a period of time and then fall back towards the centre to a lower level.
- The energy it absorbed when moving out will be released in the form of electromagnetic radiation. The quantity of energy released determines the frequency of the radiation or the colour of the light.
- Every element has a characteristic mix of electrons and therefore, electrons tend to move between the same levels within those elements. This means that each element has its own characteristic spectrum, which always consists of the same mix of colours.